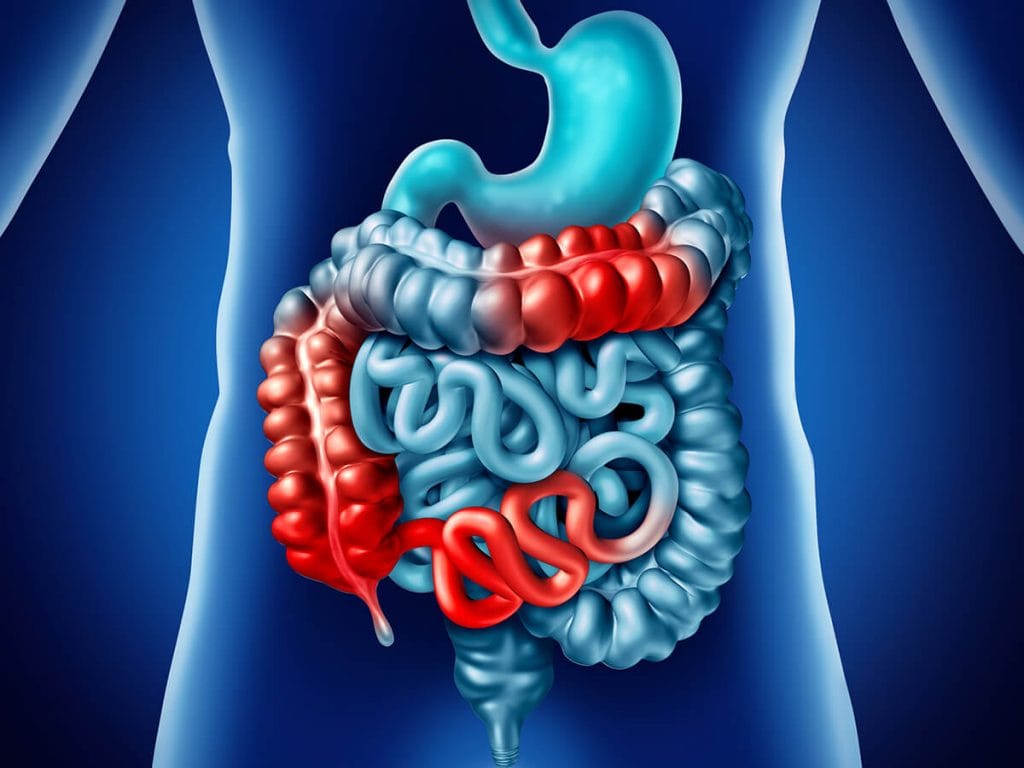
Inflammatory Bowel Disease (IBD) refers to an inflammatory condition of the digestive tract (gut) resulting from abnormal immune system activity. It manifests in two distinct forms: Ulcerative Colitis (UC) and Crohn’s disease. UC primarily involves inflammation of the rectum, the terminal segment of the colon preceding the anus. Over time, the inflammation can extend throughout the entire colon. Crohn’s typically affects the area where the small intestine and large intestine converge, known as the ileocecal valve, although it can impact any part of the digestive tract spanning from the mouth to the anus.
Both UC and Crohn’s are chronic ailments characterized by recurring episodes. Periods of active symptoms, known as flares, alternate with periods of remission or reduced symptoms. Many individuals necessitate ongoing IBD treatments to effectively manage symptoms and mitigate chronic inflammation.
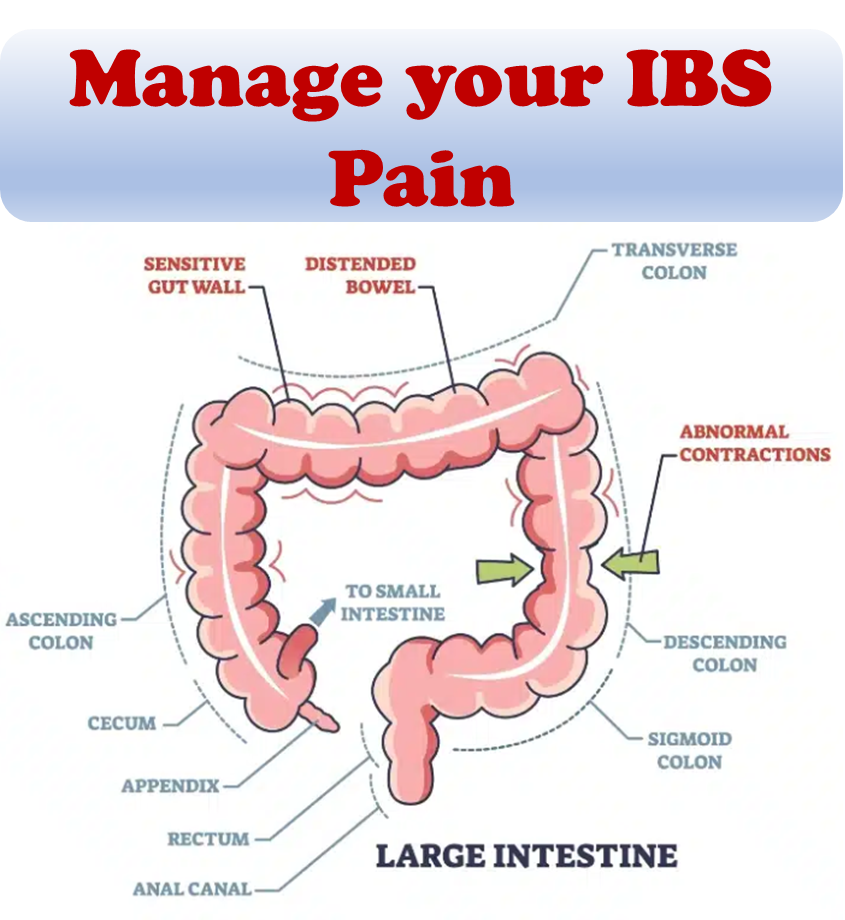
Around one-third of individuals with IBD experience persistent gastrointestinal symptoms similar to irritable bowel syndrome (IBS) in the absence of objective evidence of disease activity. The cause of these IBS-like symptoms is unclear, but it has been suggested that changes in the gut-brain axis, epithelial barrier dysfunction, and the gut flora may be partially responsible.
While patients of IBD do have an increased risk of colorectal cancer, this is usually caught much earlier than the general population in routine surveillance of the colon by colonoscopy, and therefore patients are much more likely to survive.
The goal of IBD treatments is toward achieving remission, after which the patient is usually switched to a lighter drug with fewer potential side effects. Every so often, an acute resurgence of the original symptoms may appear; this is known as a “flare-up”. Depending on the circumstances, it may go away on its own or require medication. The time between flare-ups may be anywhere from weeks to years, and varies wildly between patients – a few have never experienced a flare-up.
What causes IBD?
The immune system’s involvement in IBD remains somewhat enigmatic, as the precise mechanisms are not yet fully understood. Variances in symptoms between UC and Crohn’s are likely attributed to distinctive inflammatory patterns.
Within UC, inflammation primarily affects the inner layer of the gastrointestinal tract. Conversely, Crohn’s entails inflammation that spans the entire thickness of the gut wall, leading to the potential development of fistulas connecting the intestines to neighboring organs. Ongoing research into potential causes of IBD encompasses:
- Genetic factors, particularly genes associated with immune system functionality.
- Environmental influences, including dietary choices.
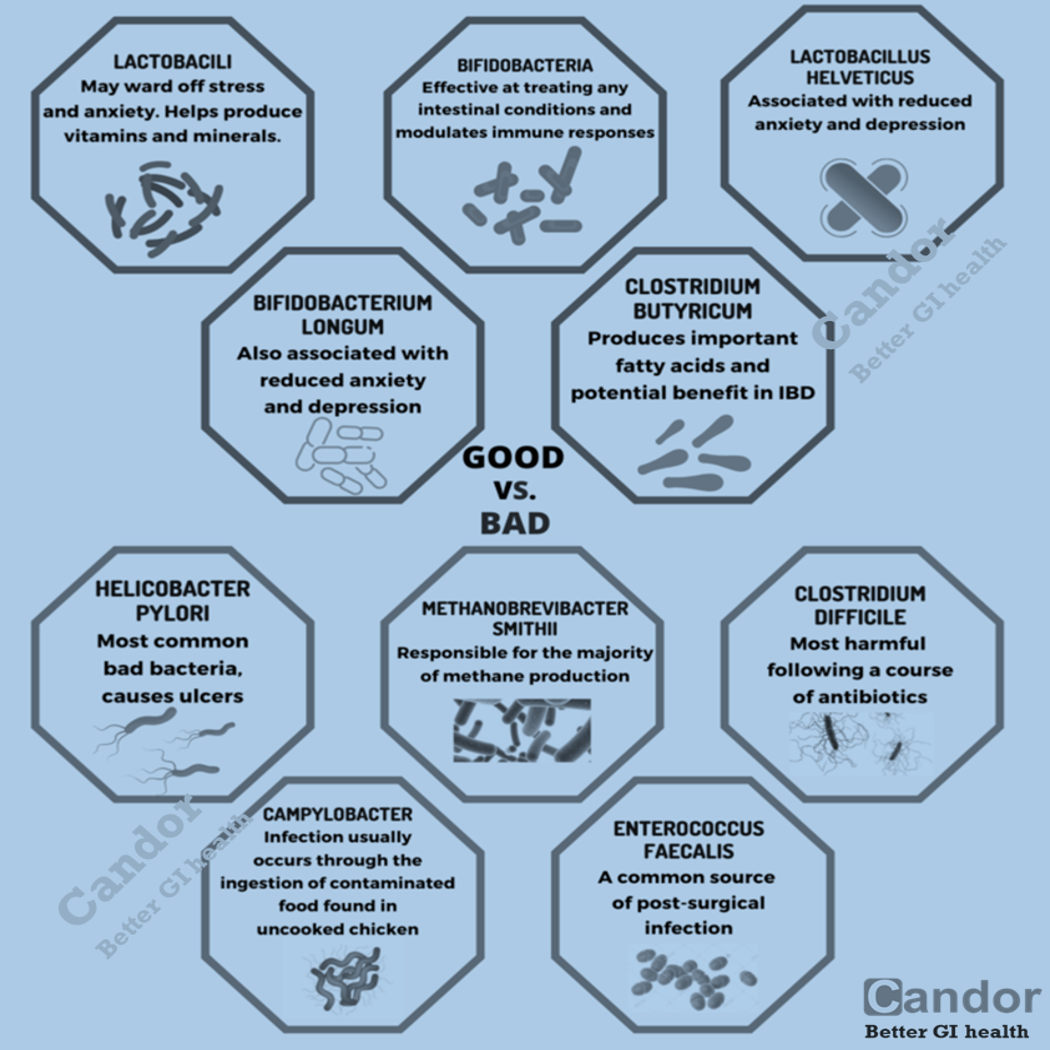
- The composition of normal gut flora and other microorganisms, collectively known as the “microbiome.”
Impact of diet
Patients should be encouraged to adopt diets that are best supported by evidence and involve monitoring for the objective resolution of inflammation. Gluten sensitivity is common in IBD and associated with having flareups. Gluten sensitivity was reported in 23.6% and 27.3% of Crohn’s disease and ulcerative colitis patients, respectively. A diet high in protein, particularly animal protein, and/or high in sugar may be associated with increased risk of inflammatory bowel disease and relapses.
Impact of microbiota
As a result of microbial symbiosis and immunity, alterations in the gut microbiome may contribute to inflammatory gut diseases. IBD-affected individuals have been found to have 30–50% reduced biodiversity of commensal bacteria, such as decreases in Bacillota (namely Lachnospiraceae) and Bacteroidota. Further evidence of the role of gut flora in the cause of inflammatory bowel disease is that IBD-affected individuals are more likely to have been prescribed antibiotics in the 2–5 year period before their diagnosis than unaffected individuals. The enteral bacteria can be altered by environmental factors, such as concentrated milk fats (a common ingredient of processed foods and confectionery) or oral medications such as antibiotics and oral iron preparations. The mucosal microbiota in the large intestine of IBD patients with active inflammation was found to be associated with pro-inflammatory changes to the host epigenome. However, large international studies have failed to identify a single microbial biomarker of IBD indicating it’s not driven by any single micro-organism.
Impact of intestinal barrier
Loss of integrity of the intestinal epithelium plays a key pathogenic role in IBD. Dysfunction of the innate immune system as a result of abnormal signaling through immune receptors called toll-like receptors (TLRs)—which activates an immune response to molecules that are broadly shared by multiple pathogens—contributes to acute and chronic inflammatory processes in IBD colitis and associated cancer. Changes in the composition of the intestinal microbiota are an important environmental factor in the development of IBD. Detrimental changes in the intestinal microbiota induce an inappropriate (uncontrolled) immune response that results in damage to the intestinal epithelium. Breaches in this critical barrier (the intestinal epithelium) allow further infiltration of microbiota that, in turn, elicit further immune responses. IBD is a multifactorial disease that is nonetheless driven in part by an exaggerated immune response to gut microbiota that causes defects in epithelial barrier function.
Diagnosis
To diagnose IBD, various tests are available:
- Colonoscopy: This procedure involves inserting a long, flexible camera through the anus to examine the interior of the large intestine. It allows for the collection of small tissue samples for further analysis.
- Upper Endoscopy: If Crohn’s disease is suspected, an upper endoscopy may also be performed. Similar to a colonoscopy, a device is used, but it is inserted through the mouth to visualize the esophagus, stomach, and a section of the small bowel.
- Video Capsule Endoscopy: Another option is a video capsule endoscopy, which entails swallowing a small pill camera that captures images of the digestive tract.
- Magnetic Resonance Enterography (MRE): This specialized imaging study provides detailed images of the intestines and surrounding structures.
Following the diagnosis of UC or Crohn’s, the primary objective in treating IBD is to eliminate underlying inflammation within the gut and promote healing, commonly referred to as achieving “remission.” A variety of medications can be employed to achieve this goal.
Medication adjustments may be made to maintain remission and prevent further inflammation. It’s important to note that most individuals with IBD will experience recurring symptoms, known as “flares.” In severe cases, surgery may be necessary to remove affected segments of the bowel.
The diagnosis is usually confirmed by biopsies on colonoscopy. Fecal calprotectin is useful as an initial investigation, which may suggest the possibility of IBD, as this test is sensitive but not specific for IBD. No disease specific markers are currently known in the blood, enabling the reliable separation of Crohn’s disease and ulcerative colitis patients. The way doctors can tell the difference between Crohn’s disease and UC is the location and nature of the inflammatory changes. Crohn’s can affect any part of the gastrointestinal tract, from mouth to anus (skip lesions), although a majority of the cases start in the terminal ileum. Ulcerative colitis, in contrast, is restricted to the colon and the rectum. Microscopically, ulcerative colitis is restricted to the mucosa (epithelial lining of the gut), while Crohn’s disease affects the full thickness of the bowel wall (“transmural lesions”). Lastly, Crohn’s disease and ulcerative colitis present with extra-intestinal manifestations (such as liver problems, arthritis, skin manifestations and eye problems) in different proportions.
IBD in children
Inflammatory Bowel Disease (IBD) can affect children as well. The symptoms of ulcerative colitis (UC) in children closely resemble those experienced by adults. However, in the case of Crohn’s disease (CD), initial symptoms in children may be more inconspicuous compared to adults. One notable indicator in children could be weight loss, or you might observe a deceleration in their growth rate, along with a potential decline in muscle mass, evident through thinner limbs.
IBD treatments
Surgery
CD and UC are chronic inflammatory diseases, and are not medically curable. However, ulcerative colitis can in most cases be cured by proctocolectomy, although this may not eliminate extra-intestinal symptoms. An ileostomy will collect feces in a bag. Alternatively, a pouch can be created from the small intestine; this serves as the rectum and prevents the need for a permanent ileostomy. Between one-quarter and one-half of patients with ileo-anal pouches do have to manage occasional or chronic pouchitis.
Surgery cannot cure Crohn’s disease but may be needed to treat complications such as abscesses, strictures or fistulae. Severe cases may require surgery, such as bowel resection, strictureplasty or a temporary or permanent colostomy or ileostomy. In Crohn’s disease, surgery involves removing the worst inflamed segments of the intestine and connecting the healthy regions, but unfortunately, it does not cure Crohn’s or eliminate the disease. At some point after the first surgery, Crohn’s disease can recur in the healthy parts of the intestine, usually at the resection site.
Medical therapies
Medical IBD treatments iare individualized to each patient. The choice of which drugs to use and by which route to administer them (oral, rectal, injection, infusion) depends on factors including the type, distribution, and severity of the patient’s disease, as well as other historical and biochemical prognostic factors, and patient preferences. For example, mesalazine is more useful in ulcerative colitis than in Crohn’s disease. Generally, depending on the level of severity, IBD may require immunosuppression to control the symptoms, with drugs such as prednisone, tumor necrosis factor inhibitors (TNF inhibitors), azathioprine, methotrexate, or 6-mercaptopurine.
Steroids, such as the glucocorticoid prednisone, are frequently used to control disease flares and were once acceptable as a maintenance drug. Biological therapy for inflammatory bowel disease, especially the TNF inhibitors, are used in people with more severe or resistant Crohn’s disease and sometimes in ulcerative colitis.
Treatment is usually started by administering drugs with high anti-inflammatory effects, such as prednisone. Once the inflammation is successfully controlled, another drug to keep the disease in remission, such as mesalazine in UC, is the main treatment. If further treatment is required, a combination of an immunosuppressive drug (such as azathioprine) with mesalazine (which may also have an anti-inflammatory effect) may be needed, depending on the patient. Controlled release budesonide is used for mild ileal Crohn’s disease.
Nutritional and dietetic therapies
Nutritional deficiencies play a prominent role in IBD. Malabsorption, diarrhea, and GI blood loss are common features of IBD. Deficiencies of B vitamins, fat-soluble vitamins, essential fatty acids, and key minerals such as magnesium, zinc, and selenium are extremely common and benefit from replacement therapy. Dietary interventions, including certain exclusion diets like the specific carbohydrate diet (SCD) can be beneficial for symptom management. Dietary fiber interventions, such as psyillium supplementation (a mixture of soluble and insoluble fibers), may relieve symptoms as well as induce/maintain remission by altering the microbiome composition of the GI tract, thereby improving regulation of immune function, reducing inflammation, and helping to restore the intestinal mucosal lining.
Anemia is commonly present in both ulcerative colitis and Crohn’s disease. Due to raised levels of inflammatory cytokines which lead to the increased expression of hepcidin, parenteral iron is the preferred treatment option as it bypasses the gastrointestinal system, has lower incidence of adverse events and enables quicker treatment.
Gastrointestinal bleeding, occurring especially during ulcerative colitis relapse, can contribute to anaemia when chronic, and may be life-threatening when acute. To limit the possible risk of dietary intake disturbing hemostasis in acute gastrointestinal bleeding, temporary fasting is often considered necessary in hospital settings.
IBD treatments through microbiome
There is preliminary evidence of an infectious contribution to inflammatory bowel disease in some patients that may benefit from antibiotic therapy, such as with rifaximin. The evidence for a benefit of rifaximin is mostly limited to Crohn’s disease with less convincing evidence supporting use in ulcerative colitis.
The use of oral probiotic supplements to modify the composition and behaviour of the microbiome has been considered as a possible therapy for both induction and maintenance of remission in people with Crohn’s disease and ulcerative colitis. For ulcerative colitis, there is low-certainty evidence that probiotic supplements may increase the probability of clinical remission.
Life with IBD can be challenging; however, many with the condition lead relatively normal lives. IBD carries a psychological burden due to stigmatization of being diagnosed, leading to high levels of anxiety, depression, and a general reduction in the quality of life.
References:
- Baumgart DC, Carding SR (May 2007). “Inflammatory bowel disease: cause and immunobiology”. Lancet.
- Xavier RJ, Podolsky DK (July 2007). “Unravelling the pathogenesis of inflammatory bowel disease”. Nature.
- Limketkai, Berkeley N.; Hamideh, Mohamed; Shah, Rishabh; Sauk, Jenny S.; Jaffe, Nancee (2022-01-29). “Dietary Patterns and Their Association With Symptoms Activity in Inflammatory Bowel Diseases”. Inflammatory Bowel Diseases.
- Charlebois A, Rosenfeld G, Bressler B (June 2016). “The Impact of Dietary Interventions on the Symptoms of Inflammatory Bowel Disease: A Systematic Review”. Critical Reviews in Food Science and Nutrition.
- Limketkai, Berkeley N; Akobeng, Anthony K; Gordon, Morris; Adepoju, Akinlolu Adedayo (2020-07-17). Cochrane Gut Group (ed.). “Probiotics for induction of remission in Crohn’s disease”. Cochrane Database of Systematic Reviews.
- Wikipedia article on IBD https://en.wikipedia.org/wiki/Inflammatory_bowel_disease